LEBREXIA-STROKE
/
Info coming soon
ARCADIA
/To determine if apixaban is superior to aspirin for the prevention of recurrent stroke in patients with cryptogenic ischemic stroke and atrial cardiopathy, and to determine if the relative efficacy of apixaban over aspirin increases with the severity of atrial cardiopathy.
TIMELESS
/This study will evaluate the efficacy and safety of tenecteplase compared with placebo in participants with acute ischemic stroke.
DOCUMENTS:
Pocket card
(contains all information: I&E, screening/randomization, checklists, etc)
INFORMED CONSENT:
MOST
/The goal of this study is to determine the effectiveness of combining tPA with blood thinners argatroban or eptifibatide. The research will look at whether combining tPA with either argatroban or eptifibatide will produce better results in stroke patients in the first 90 days after suffering a stroke versus tPA with a placebo.
DOCUMENTS:
eConsent LinkS:
UCMC: https://redcap.link/ucmcmost
Jewish: https://redcap.link/MOSTmercyjewish
Mercy West: https://redcap.link/MOSTmercywest
Mercy Anderson: https://redcap.link/MOSTmercyanderson
ENDOLOW
/This study will test the hypothesis that patients presenting within 8 hours of onset with cerebral ischemia in the setting of proximal LVO and low baseline NIHSS scores (0-5) will have better 90-day clinical outcomes (mRS distribution) with immediate mechanical thrombectomy (iMT) compared to initial medical management (iMM).
STEP
/
The master protocol defines the core principles of the design and operation for all sites and all domains of the STEP platform. The Master Protocol may be amended but it is anticipated that such amendments will be infrequent.
Domain-Specific Appendices contain only that information specific to those interventions within a domain and not already specified in the master protocol. As such, the Master Protocol does not include information about the intervention(s) that will be evaluated within the platform, but rather provides the framework on which multiple different interventions, within domains, can exist within this trial.
To determine subsets of AIS patients within 24 hours of last known well, currently not treated with EVT according to guidelines, who do or do not benefit from EVT compared to Medical Management by having better functional outcome.
NOTE: As of 3/11/25 we will EXCLUDE tandem occlusions and exclude known ICAD.
CAPTIVA
/The primary goal of the trial is to determine if the experimental arms (rivaroxaban or ticagrelor or both) are superior to the clopidogrel arm for lowering the 1-year rate of ischemic stroke, intracerebral hemorrhage, or vascular death.
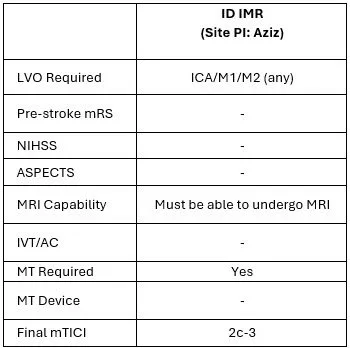
ARPEGGIO and ID IMR
/
Call Yazmin 412-913-8424
For stroke patient with anterior LVO is going to IR or being considered for IR when you are on stroke call. We have a script ready to share with patients and can engage the appropriate CRCs accordingly. You do NOT need to know the I/E criteria provided below, we are just sharing for convenience sake:
ASPIRE
/To determine if apixaban is superior to aspirin for prevention of the composite outcome of any stroke or death from any cause in patients with recent ICH and atrial fibrillation. Secondary Aim: To determine if apixaban, compared with aspirin, results in better functional outcomes as measured by the modified Rankin Scale.
DOCUMENTS:
INFORMED CONSENT:
MEDIA:
SISTER
/SISTER is a phase-2, prospective, randomized, placebo-controlled, blinded, dose-finding trial that aims to determine the safety and preliminary efficacy of TS23. TS23 is a monoclonal antibody against the alpha-2 antiplasmin (α2-AP), in acute ischemic stroke.
The study will be conducted at up to 50 U.S. sites and enroll 300 adults (>18 years) with acute ischemic stroke who have a baseline NIHSS >6 and are able to receive study drug within 4.5-24 hours after stroke onset (or last known well) who have evidence of core-penumbra mismatch on baseline perfusion imaging and are not planned for endovascular intervention or standard of care intravenous (IV) thrombolysis.
TESTED
/The overall goal of the TESTED study, we will compare the risks and benefits of EVT to medical management (no EVT) in ischemic stroke patients who have a blockage in one of the large blood vessels in the brain and have a moderate-to-severe disability prior to their stroke.
DISCOVERY
/The overall goal of the DISCOVERY study is to better understand what factors contribute to changes in cognitive (i.e., thinking and memory) abilities in patients who experienced a stroke. The purpose of the study is to help doctors identify patients at risk for dementia (decline in memory, thinking and other mental abilities that significantly affects daily functioning) after their stroke so that future treatments may be developed to improve outcomes in stroke patients.
DOCUMENTS:
INFORMED CONSENT:
MISC:
VERIFY
/VERIFY will validate biomarkers of upper extremity (UE) motor outcome in the acute ischemic stroke window for immediate use in clinical trials, and explore these biomarkers in acute intracerebral hemorrhage.
DOCUMENTS:
INFORMED CONSENT:
MEDIA:
Sleep SMART
/The primary goals of this study are to determine whether treatment of obstructive sleep apnea (OSA) with positive airway pressure starting shortly after acute ischemic stroke or high risk TIA (1) reduces recurrent stroke, acute coronary syndrome, and all-cause mortality during 6 months after the event, and (2) improves stroke outcomes at 3 months in patients who experienced an ischemic stroke.
DOCUMENTS:
INFORMED CONSENT:
SATURN
/This study focuses on individuals who have had a brain hemorrhage and were taking a statin drug. The purpose of the study is to determine whether it is better to continue or discontinue statin drugs in patients who had a brain hemorrhage while taking a statin drug.
CREST-2
/CREST-2 is designed to compare three different methods of stroke prevention to find the safest and most effective treatment: intensive medical management alone compared to intensive medical management in combination with carotid enarterectomy and carotid stenting.
FASTEST
/The objective of the FASTEST Trial is to establish the first treatment for acute spontaneous ICH within a time window and subgroup of patients that is most likely to benefit. Hypothesis is that rFVIIa, administered within 120 minutes from stroke onset with an identified subgroup of participants most likely to benefit, will improve outcomes at 180 days as measured by the mRS and decrease ongoing bleeding as compared to standard therapy.